Group-sequential and adaptive designs in immuno-oncology
Abstract
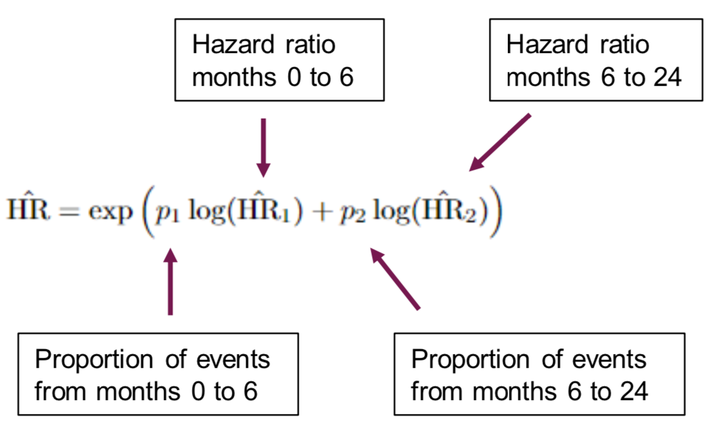
Here I talk about designing a clinical trial for an immuno-oncology endpoint where we expect a delay in the seperation of the survival curves. Starting with the properties of a standard design under non-proportional hazards, I believed I had made a great improvement by adapting the sample size based on a “ratio of hazard ratios”. However, a well-chosen group-sequential design made an equal (slightly better) improvement to the operating characteristics, and is much simpler.
Date
Jun 4, 2018 2:00 PM
Event
PSI Conference 2018
Location
Amsterdam